Who we are
Biz Kimiz
Rare Meets Responsibility
Nadir, sorumluluk ister
At Trispera, we believe that serving patients with rare diseases is not only a scientific challenge, but also a moral duty. We combine global expertise with local accountability to ensure responsible access for those who need it most.
Trispera’da, nadir hastalıklara sahip hastalara hizmet etmenin yalnızca bilimsel bir zorluk değil, aynı zamanda ahlaki bir sorumluluk olduğuna inanıyoruz. En çok ihtiyacı olanlara sorumlu erişimi sağlamak için küresel uzmanlığı yerel sorumlulukla birleştiriyoruz.

Focused on Rare Diseases
Nadir Hastalıklara Odaklı
Our goal is to ensure and ease access to rare disease medicines to the patients
Hedefimiz, nadir hastalık ilaçlarına hastaların erişimini sağlamak ve kolaylaştırmaktır

With a Commitment to Excellence
Mükemmellik Anlayışıyla
Compliance to business and health ethics with a transparent, professional, sustainable and efficient manner
Şeffaf, profesyonel, sürdürülebilir ve verimli bir yaklaşımla iş ve sağlık etiğine uyum

150
Market Reports
Pazar Analizi Raporu
15
Countries
Ülke
31
Companies
Firma
42
Products
Ürün
Our services
Hizmetlerimiz
Rare deserves more than delivery, it deserves devotion
Nadire sadece erişim değil, adanmışlık gerekir
Representation & Distributorship
Temsilcilik & Distribütörlük

-
Temsilcilik
Yurtdışı İlaç Listesi kapsamında, uluslararası ilaç firmalarına Türkiye’de yetkili temsilcileri olarak özel destek sunuyoruz.
-
Distribütörlük
Distribütörlük hizmetimiz, yurt dışından ithal edilen ilaçların Türkiye’deki yerel dağıtım kanallarına ve hastalara etkin bir şekilde ulaşmasını sağlamak üzere tasarlanmıştır.
-
Ruhsatlandırma
Trispera, ürünlerini Türkiye’de ruhsatlandırmak ve pazara sunmak isteyen uluslararası ilaç firmalarına baştan sona destek sağlamaktadır.
-
Fiyatlandırma
Başarılı pazar erişimi ve sürdürülebilir ürün konumlandırmasını sağlamak amacıyla ilaç fiyatlandırma sürecinde baştan sona destek sunuyoruz.
Crafting tailor-made pharma solutions in rare diseases
Nadir hastalıklarda özel farma çözümleri
Pharma Solutions
Farma Çözümleri

-
Pazara Giriş Stratejisi
Trispera olarak, genişleme hedeflerinizi desteklemek amacıyla, yalnızca veri odaklı değil, aynı zamanda yerel düzenleyici ve etik standartlarla uyumlu pazar özelinde içgörüler sunuyoruz.
-
Stratejik Değer Analizi ve İş Modeli Geliştirme
İlaç ve biyoteknoloji firmalarının ürün portföylerini, klinik anlamlılık, pazar dinamikleri ve sağlık sisteminin önceliklerini bütüncül bir yaklaşımla analiz ederek stratejik açıdan değerlendirmelerine yardımcı oluyoruz.
-
Pazar Araştırması, Rekabet Analizi ve Fizibilite Çalışmaları
Trispera, tıbbi ve farmasötik alanlarda zamanında ve hedefe yönelik araştırmalar sunarak bilinçli karar alma süreçlerini destekler.
-
Baştan Sona Ürün Lansmanı
Trispera, ürün lansmanınız için stratejik planlamadan lansman öncesi, lansman ve lansman sonrası faaliyetlerin tam olarak yürütülmesine kadar baştan sona destek sağlar.








Testimonials
Referanslarımız
See what our partners say
Ortaklarımız ne diyor?
Discover how we’ve helped our clients achieve their goals.
Başarı hikâyelerimizi keşfedin.
FAQ
SSS
Frequently Asked Questions
Sık Sorulan Sorular
What services do you offer?

We offer three core services: Representation, providing strategic support and local presence for international partners; Distributorship, ensuring compliant and efficient market access; and Pharma Solutions, delivering tailored services for pharmaceutical companies focused on rare diseases.
Hangi hizmetleri sunuyorsunuz?

Üç temel hizmet sunuyoruz: Temsilcilik, uluslararası iş ortaklarımıza stratejik destek ve yerel varlık sağlar; Distribütörlük, mevzuata uyumlu ve verimli pazar erişimini güvence altına alır; Farma Çözümleri, nadir hastalıklara odaklanan ilaç şirketleri için özelleştirilmiş hizmetler sunar.
Do you focus only on rare diseases?

While our core expertise lies in rare diseases, we also support pharmaceutical products in other specialized or underserved therapeutic areas. Our capabilities are particularly well-suited for innovative therapies that require tailored market entry, regulatory navigation, and strategic representation.
Odağınızda yalnızca nadir hastalıklar mı var?

Temel uzmanlığımız nadir hastalıklarda olsa da, özel ya da hizmet açığı bulunan diğer terapötik alanlardaki ilaçları da destekliyoruz. Özellikle, özelleştirilmiş pazara giriş planı, düzenleyici süreçlerde etkin ilerleme ve stratejik temsil gerektiren yenilikçi tedaviler için yetkinliklerimiz son derece uygundur.
Which countries or regions do you operate in?

We are headquartered in Türkiye and primarily operate within the Turkish pharmaceutical market, as well as across the broader region including the Turkic states and Gulf countries. Leveraging our deep local expertise and extensive regional network, we guide international partners in navigating diverse healthcare ecosystems, regulatory environments, and clinical landscapes with a strong focus on rare and specialty diseases.
Hangi ülkelerde veya bölgelerde faaliyet gösteriyorsunuz?

Merkezimiz Türkiye’dedir ve öncelikli olarak Türkiye ilaç pazarında faaliyet gösteriyoruz; bunun yanında Türk Devletleri ve Körfez ülkeleri dâhil geniş bölgede de çalışıyoruz. Derin yerel uzmanlığımız ve geniş bölgesel ağımızdan yararlanarak, nadir ve özel hastalıklara güçlü odakla uluslararası iş ortaklarımıza farklı sağlık ekosistemleri, düzenleyici ortamlar ve klinik dinamikler içinde yol almalarında rehberlik ediyoruz.
How do we start working together?

We begin with an introductory meeting to understand your goals, product profile, and target market needs. Based on this, we propose a tailored collaboration model, whether for representation, distributorship, or pharma solutions. Once aligned, we proceed with due diligence, define the scope of services, and formalize our partnership through a clear and compliant agreement.
Birlikte çalışmaya nasıl başlarız?

Öncelikle hedeflerinizi, ürün profilinizi ve hedef pazar ihtiyaçlarını anlamak için bir tanışma toplantısı yapıyoruz. Bu doğrultuda temsilcilik, distribütörlük veya farma çözümleri kapsamında size özel bir iş birliği modeli öneriyoruz. Uzlaşı sağlandıktan sonra due diligence (durum tespiti) sürecini yürütüyor, hizmet kapsamını netleştiriyor ve iş birliğimizi açık, şeffaf ve mevzuata uygun bir sözleşmeyle resmileştiriyoruz.
Blog
Highlights from the blog
Öne Çıkan Yazılar
-

Trispera CEO’su ile Röportaj
Hastalara ve hasta yakınlarına dolaylı olarak destek olmak arzusuyla hem de bu alanda gördüğümüz karşılanmamış ihtiyacı giderebilmek amacıyla Trispera Pharma Solutions olarak nadir hastalıklar alanında fayda oluşturmaya odaklandık.
-

Trispera Medikal Direktörü Dr. Ekin Öztüzün ile Röportaj
İlaç şirketlerinin ihtiyaçlarına özel çözümlerimizle, regülasyonlara uygunluk, pazar erişimi ve stratejileri konusunda danışmanlık sağlıyoruz ve ilaç şirketlerinin hastalara hayat kurtaran tedavileri ulaştırmalarını sağlamayı hedefliyoruz.
-
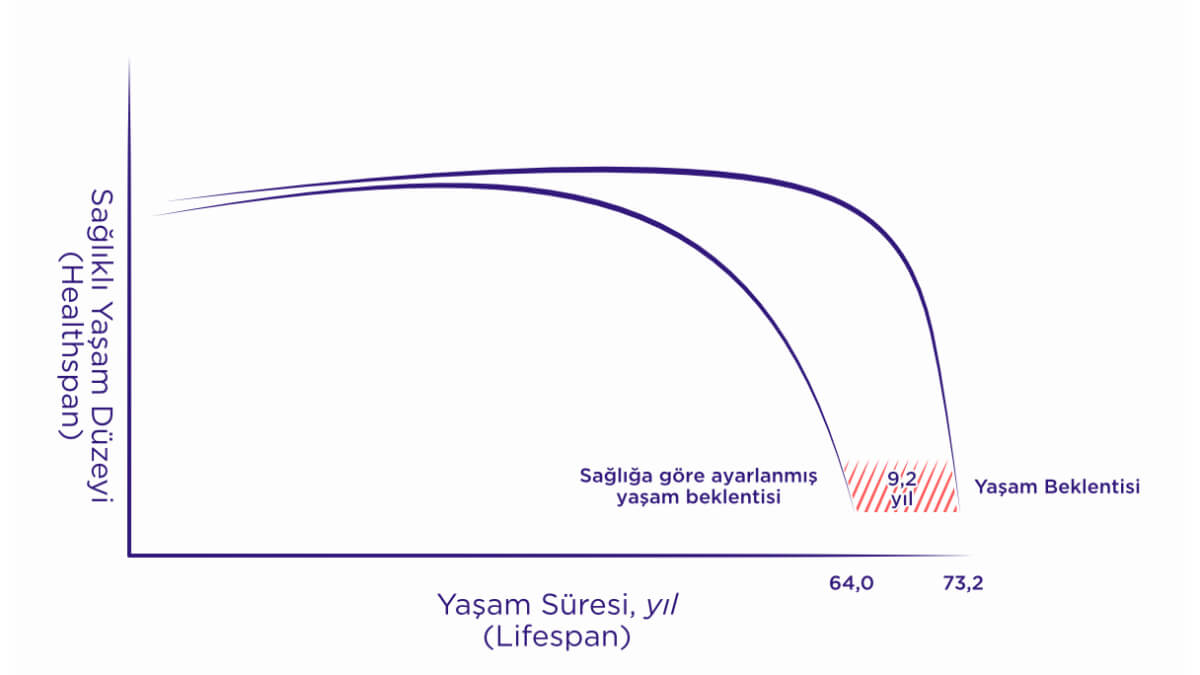
Lifespan, Healthspan, Financialspan
Günümüzde on milyonlarca insanı etkileyen, tüm kronik hastalıkların ortak artış sebebi yaşlanma olarak bilinmekte, dünya çapında ekonomilere ağır bir yük oluşturmaktadır.
Driven by Expertise, Defined by Results
Gücümüz uzmanlığımız, farkımız sonuçlarımız
With expertise in rare diseases and complex healthcare challenges, Trispera builds trusted partnerships to advance meaningful progress.
Trispera, nadir hastalıklar ve karmaşık sağlık süreçlerindeki uzmanlığını güvenilir iş ortaklıklarına dönüştürerek somut ve değer üreten ilerleme sağlar.






